- 400-820-2919
- CN

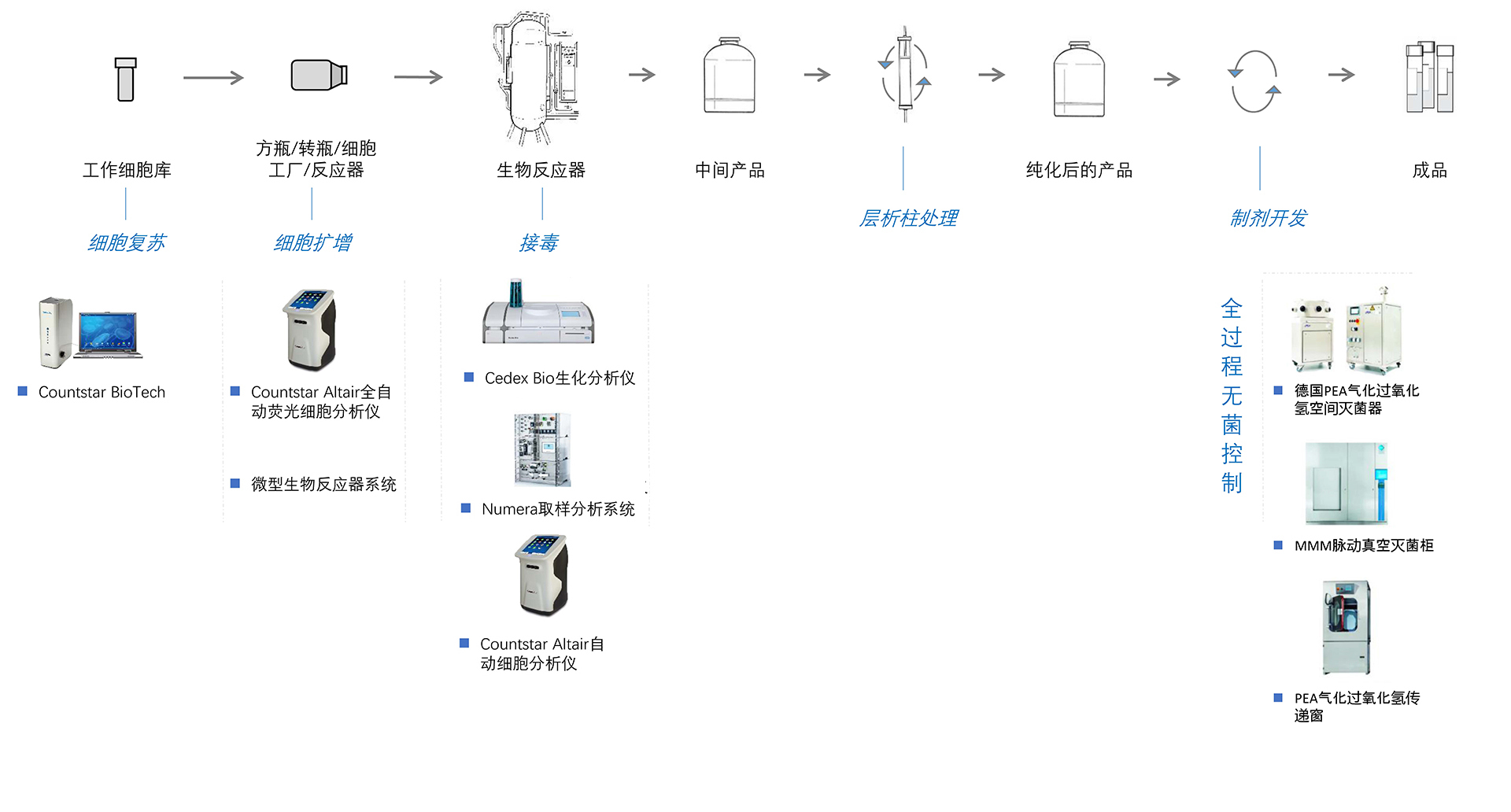
After the vaccine incident, the state requiresvaccine enterprises to carry out standardized production more strictly inaccordance with Drug Administration Law of the people's Republic of China, Goodmanufacturing practice (GMP) and Registration standard requirements to ensurethe quality control of vaccine production.
After a short decline in China's vaccine market,with the opening of the two-child policy, the growth of the number of newborns,the upgrading of consumption, the more standardized vaccine industry, theR&D cycle of the vaccine industry and other favorable factors, as well asthe launch of new heavy-duty vaccines, such as 7-valent、13-valent pneumonia vaccines and HPV vaccines,the domestic vaccine industry has recovered stable growth.
Alit Life Science has been deeply involved inthe vaccine industry for many years. Its independently developed Countstarseries cell analyzer meets the requirements of high-tech R&D and GMPproduction. At the same time, Alit Life Science integrates advanced vaccineprocess equipment solutions, the whole process aseptic control solutions andtechnical services in Europe and America so as to provide you withinternational advanced high-quality compliance process equipment solutions andtechnical services, and reduce operational and risk costs, increase industrycompetitiveness for you.


Scan the code, pay attention to Wechat Public Number and get the latest information
Clean up and pay attention to it immediately