- 400-820-2919
- CN

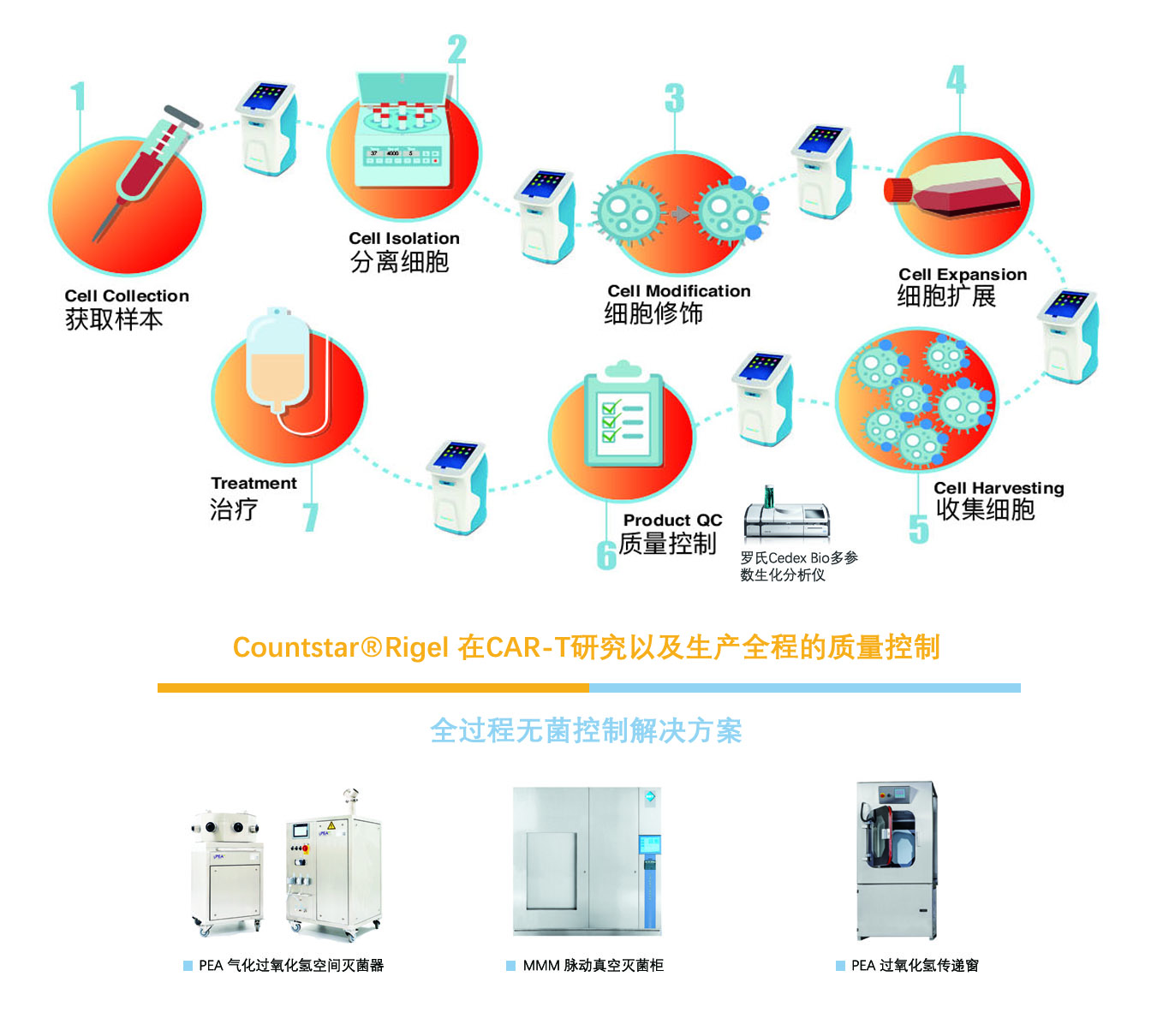
At present, car-t immunocytotherapytechnology is in the stage of rapid development. At the end of 2017, the StateFood and Drug Administration successively issued the measures for theadministration of drug registration (Revised Version) (for comments), guidingprinciples for research and evaluation technology of cell therapy products (forTrial Implementation), and other relevant regulations. In June 2018, China foodand drug inspection and Research Institute issued car-t The key points ofquality control and clinical research of cell therapy products. Theseregulations have made it clear that cell therapy should be approved inaccordance with drugs, preliminarily standardized and guided the research andevaluation of cell therapy products.
Under the guidance of the new policy, domesticcell therapy R & D enterprises have launched preclinical research and INDapplication of new drugs. Since Nanjing legend submitted the clinicalapplication of car-t as the first class I new drug, more than 10 enterprisesand more than 20 varieties have successively received the acceptance of CDE,and more than 10 enterprises have received the acceptance of stem cell therapy.Cell therapy has made great progress. Cell therapy itself is no longer a simplecell collection and reinfusion. Countstar products have been accepted by manyleading cell therapy companies at home and abroad. We can help our customersestablish a stable and reliable cell concentration activity monitoring system.Countstar is committed to the research and development of modern cell analysistechnology and instrument manufacturing, providing professional cell qualitycontrol solutions for cell therapy customers, and escorting cell products.
At the same time, the scale of the cellpreparation is small, but the individual differentiation is strong, there aremany batches of simultaneous characteristics, easy to occur pollution and crosspollution; different from the traditional sterile preparation, the cellpreparation can not be sterilized and filtered in the whole process, and thereare many operations in the incomplete sealed state, which puts forward morestringent requirements for space sterilization and sterilization of articles. PEAgasified hydrogen peroxide sterilization equipment in Germany is a movablesterilization equipment, which can easily and flexibly meet the sterilizationneeds of customers, and more conform to the laws and regulations in terms ofdata integrity, authority management and audit tracking. MMM pulsating vacuumsterilizer in Germany is a flexible and diversified sterilizer suitable forimmunotherapy industry, which meets the requirements of FDA 21 CFR Part 11 andGMP. It can be adjusted according to the user's functions, including equipmentand software. It can provide sterilization cabinets from 20L to 7000l invarious volumes, such as damp heat, dry heat, steam air mixing, water bathspray, etc. The MMM pulsating vacuum sterilizer, pea vaporized hydrogenperoxide space sterilizer and transfer cabin in Germany provide the car-tindustry with a sterilization solution that meets the requirements ofcustomers.


Scan the code, pay attention to Wechat Public Number and get the latest information
Clean up and pay attention to it immediately