Introduction
Cell culture media development requires high-throughput in order to generate robust statisticalmodels capable of identifying the criticalcomponents that need to be optimized. Currently,there is no commercially available cell retentiondevice that can be used with high-throughputsystems (<100mL) in perfusion mode. In an effortto increase capability for perfusion mediadevelopment we evaluated the Erbi perfusionmicrobioreactor system, a 2mL working volumesystem with an embedded filtration based cellretention device. With Erbi Biosystems’ expertise inmicrofluidics and our expertise in perfusion animalcell culture, we have made significant improvementsto the system. At this time, we are able to achieve asteady state of at 50x106vc/mL with a CHOZN®GScell line producing a fusion protein and a CHOS cellline producing a mAb. It has comparable profiles tobench-top perfusion bioreactors and the resultshave been reproduced over multiple runs.
Methods
For this work, experiments were performed using aCHOZN®GS cell line producing a fusion protein(n=3) and a CHOS cell line producing a mAb (n=2)cultured in EX-CELL® Advanced HD PerfusionMedium in Erbi Biosystems perfusionmicrobioreactors (Erbi Biosystems, Massachusetts).Cells were centrifuged and concentrated to inoculateat 5x106vc/mL with 15% carry over. Perfusion wasinitiated on day 0 at a constant perfusion rate of2vvd (minimum CSPR 40pL/cell/day). Dissolvedoxygen, pH and target cell density were set at 40%,6.9+/-0.05 for the CHOZN®GS cell line, 7.1 6.9+/-0.05 for the CHOS cell line and 50*106vc/mLrespectively. Erbi perfusion microbioreactor data iscompared to a 3L Applikon® glass bioreactor(Applikon®, Netherlands) with 2L working volume,using the same cell line and medium. Cells were inoculated at 2x106 vc/mL with 50% carry over. Perfusion was initiated on day 2 with a constantCSPR of 40 pL/cell/day (perfusion rate range 0.18-2vvd). Dissolved oxygen, pH and target cell densityhad the same settings used for the Erbi perfusionmicrobioreactor experiments.
EX-CELL® Advanced HD Perfusion Medium wastested for minimum CSPR using the CHOZN®GS cellline in both the 3L Applikon glass bioreactor andErbi perfusion microbioreactor. The same protocolwas used for both systems. Once the culturesreached 50x106vc/mL, the perfusion flow rate waschanged to 1vvd (20pL/cell/day) for 5 days, andthen changed to 0.5 vvd (10pL/cell/day) thereafter.
Cell density and viability were measured using Vi-Cell®XR (Beckman-Coulter, California). Metaboliteswere analyzed using Nova®FLEX2 (Nova Biomedical,Massachusetts). Protein concentration wasmeasured using HPLC.
Results
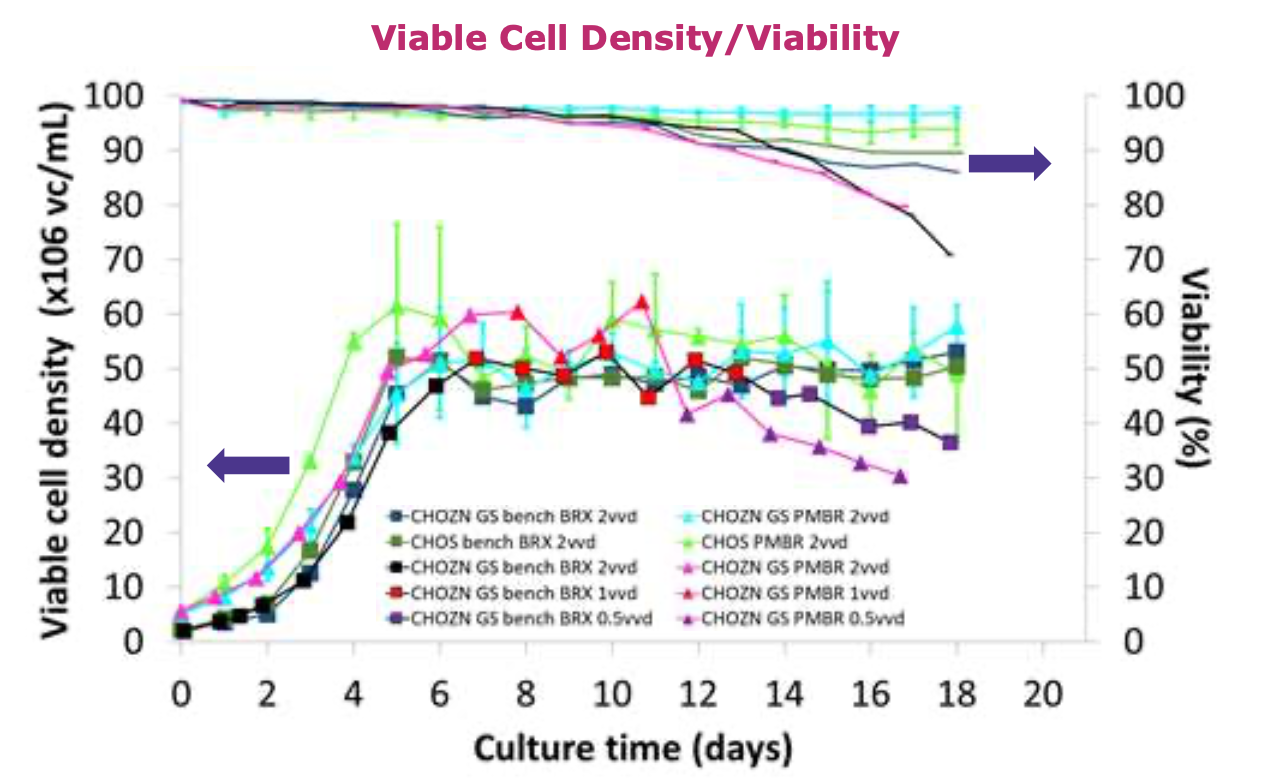
• Target cell density in the Erbi perfusion microbioreactor ismaintained through a control loop that regulates the valve on thebleed line in response to the viable cell density measured by theoptical sensors; in comparison, bench-top bioreactor’s control loopuses capacitance probes to measure viable cell density andregulates cell bleeding pumps.
• Steady state at 50x106vc/mL was achieved for both cell lines.
• Cell viability in the Erbi perfusion microbioreactor cultures was higher compared to the bench-top bioreactor.
-
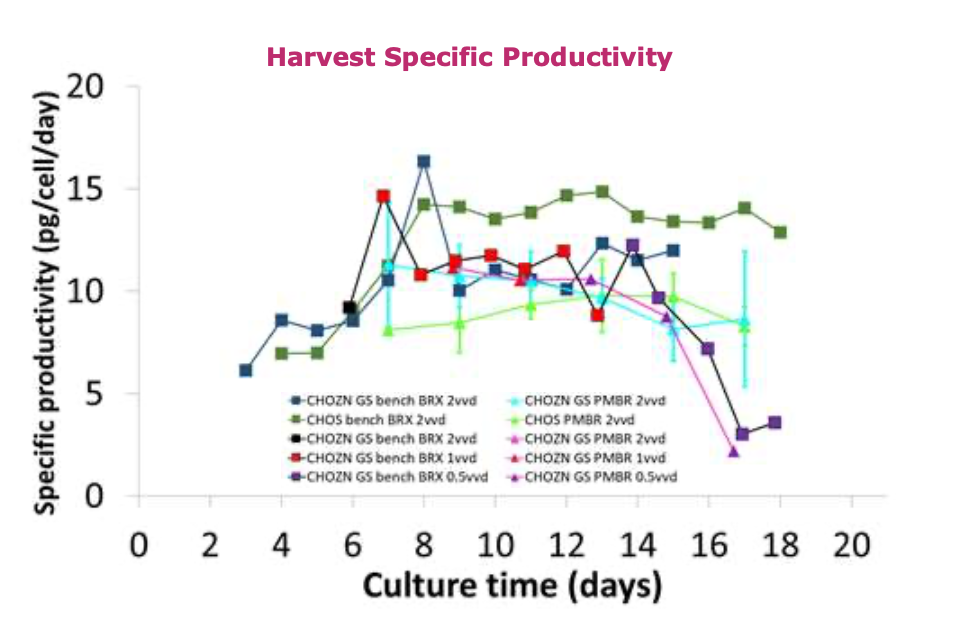
Harvest specific protein production for CHOZN®GS cell show comparable trends.
-
Differences in specific productivity observed for CHOS cell line. Limitations in daily off-line pH correction in the Erbi perfusion microbioreactor may contribute to differences.
-
Harvest specific productivity show a decrease after day 13. This correlates with higher protein retention observed in the Erbi perfusion microbioreactor cell retentions device (data not shown).
- Minimum CSPR test with CHOZN®GS cell in Erbi perfusion microbioreactor compare well with benchtop bioreactor data.
-
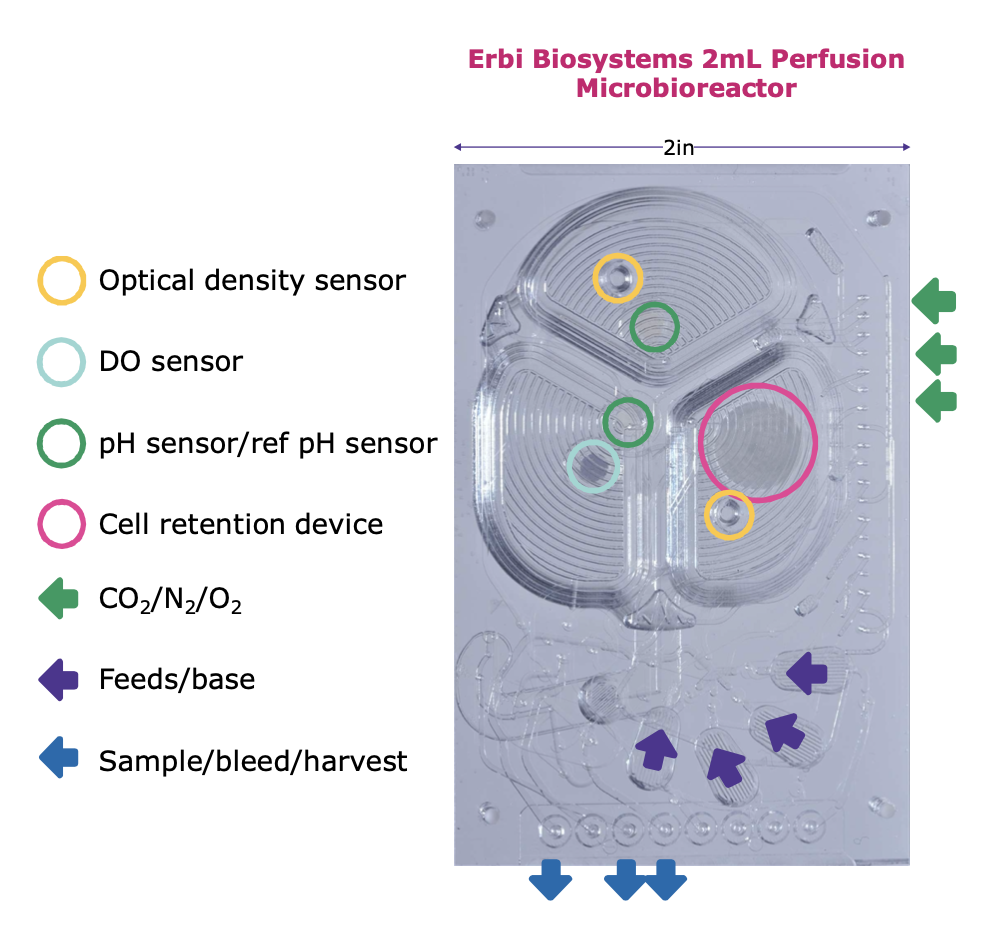
The 2mL Erbi perfusion microbioreactor contains optical sensors for cell density, dissolved oxygen and pH.
-
The growth chamber comprises three interconnected sub- chambers with a silicone membrane that can be inflated to displace the fluid in each sub-chamber.
-
By sequentially inflating the three sub-chambers, the culture is efficiently mixed and gasses for cell culture are efficiently transferred to the liquid phase by silicone membrane diffusion.
-
Fluid flow is controlled through integrated pumps and valves.
-
Fresh media can be added continuously to the culture while continuous harvest is being filtered through the cell retention device and allowing 100% cell retention in the micro-
bioreactor.
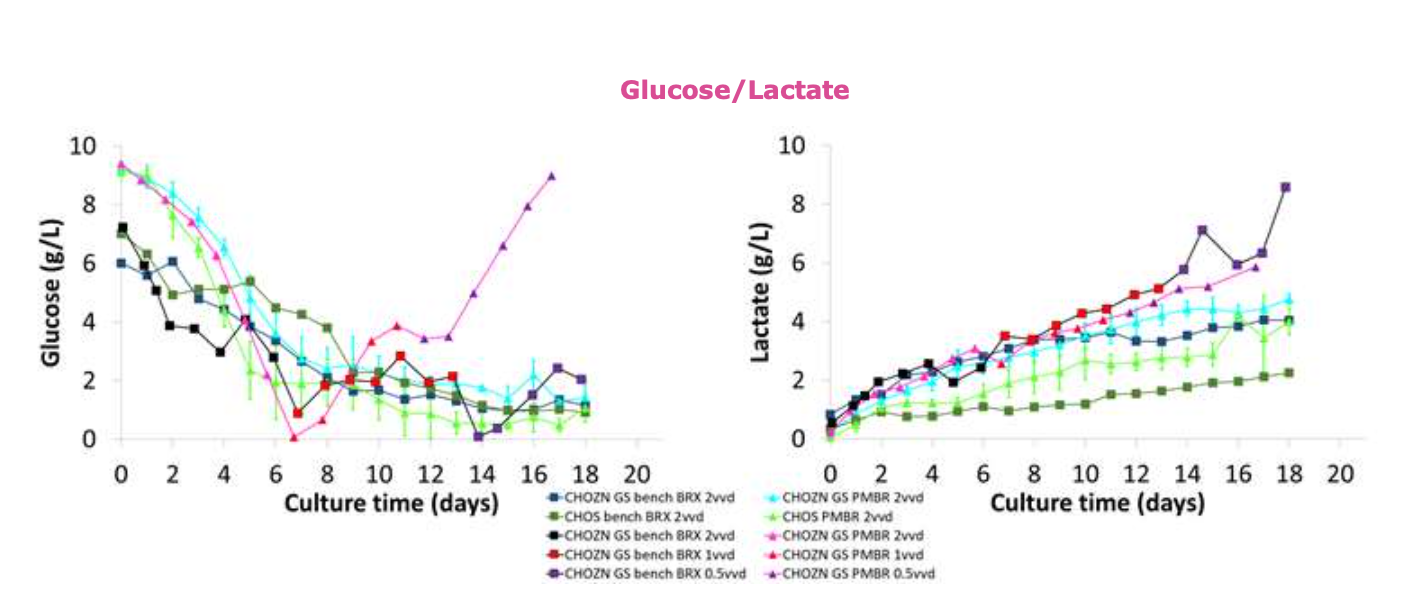
• Glucose concentrations for both cell lines show comparable results between the Erbi microbioreactor and benchtop bioreactor at 2vvd.
• Lactate concentrations were comparable for CHOZN ®GS cell line at 0.5-2vvd, but differences were observed with the CHOS cell line.
• Glucose concentrations at 0.5vvd in the CHOZN ®GS cell line show significant differences and lactate concentrations show comparability across all flow rates.
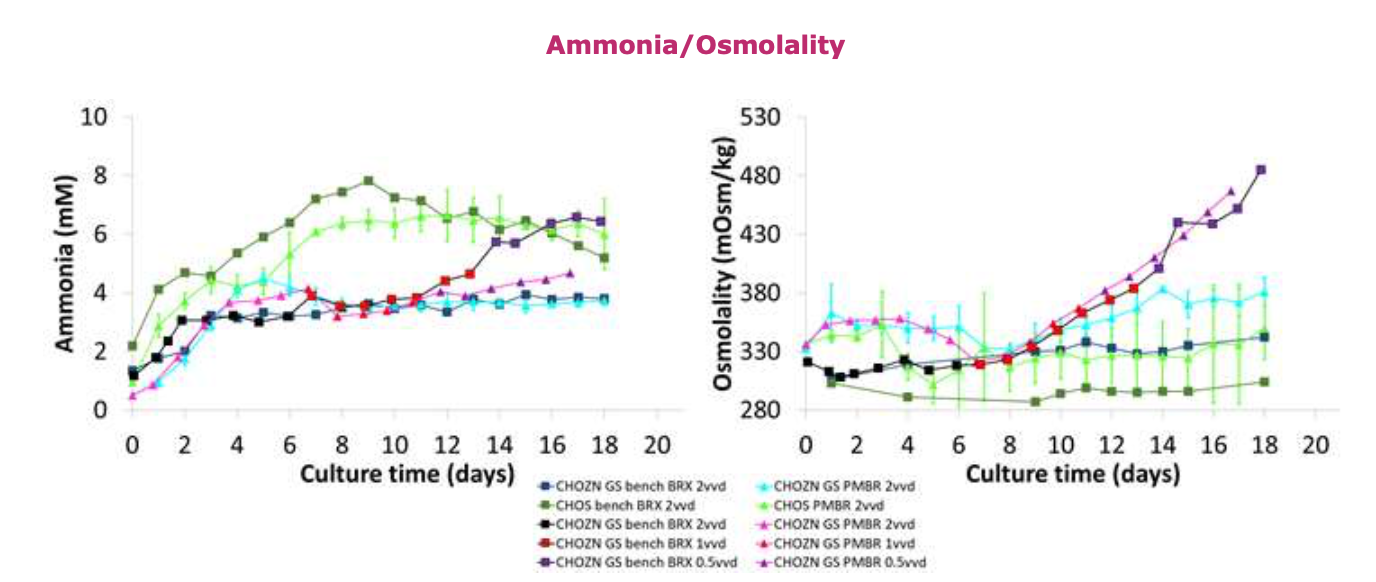
• Ammonia concentrations for both cell lines show comparable results between the Erbi microbioreactor and benchtop bioreactor at 2vvd.
• The CHOZN ®GS cell line show lower concentrations in the Erbi perfusion microbioreactor at 0.5vvd compared to the benchtop bioreactor condition.
• Differences in osmolality were observed between the Erbi perfusion microbioreactor and bench-top bioreactor with the Erbi perfusion microbioreactor showing consistently higher levels.
• Nova® FLEX2 metabolite differences between Erbi perfusion microbioreactor and bench-top bioreactor do not fully explain the differences observed in osmolality.
• Total amino acid concentration in spent media was 20mM higher (data not shown) in the Erbi perfusion microbioreactor compared to the bench-top bioreactor, which better explains the differences in osmolality.
Summary
Erbi Biosystems perfusion microbioreactor shows high potential as a small-scale model for perfusion processes. The critical characteristics that distinguish the micro-bioreactor from other small-scale models evaluated are the cell retention device, continuous fluid flow, automated cell bleeding, pH/DO control and high-throughput potential. Culture profiles were comparable to bench-top perfusion bioreactors and the results have been reproduced over multiple runs. Two critical improvements have been identified for the Erbi perfusion microbioreactor to fulfill the needs of an accurate small-scale model for continuous perfusion processes: increased working volume and an improved cell retention device.


